I made it a while ago, put it aside, then today finally added it in my "melting box". And here are the reasons why I'm not happy with it:
1. I decided to turn my learning silver parts into finished pieces. Wrong. Clueless design leads to clueless piece unless you are lucky to come out with something interesting.
2. Scratches! Lost of them.
3. Solder shows up on the wrong side, not good job.
4. Shaping. Lousy, should do better, more natural looking leaf.
5. The wire on the middle should be thicker and well shaped, bail should be thicker as well, more volume needed. The way how I shaped it su... is so amateur.
6. The whole composition is questionable.
7. All together this piece screams: I'm novice!
What do you think?





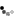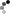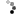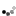
 Reply With Quote
Reply With Quote


Bookmarks